Water that contains dissolved salt = saltwater
Water that contains undissolved salt = saltwater with a pile of salt in the bottom of the bucket
couldn't you carefully pour most of the water out and when the water level is near the bottom, maybe scoop more water out with a cup. When the bucket has as little water as you can possibly have in it without removing the salt at the bottom, then you put the bucket in the sun and/or in front of a fan to evaporate the remaining water.
Then you scrape the salt off the bottom of the bucket and measure it, either for volume with milliliters in a graduated cylinder or for mass in grams on a scale.
Temperature affects the amount of salt that water can dissolve because:
At high temperatures, the heat energy causes the water molecules to vibrate and bounce around so much that extra space is created between the water molecules.
Frozen solid water (aka ice) has no heat energy. A lack of heat energy means the molecules do not have any energy to move around. They are tightly packed together.
Example: It's like dropping an empty cardboard box (nothing really happens)
room temperature liquid water - has very little heat energy. Its molecules hold themselves near each other and have a little space for dissolved salt to fit in.
Example: Like magnets, you can put several pieces of paper between them and their magnetic field is unaffected. (No difference)
heated liquid water (aka what you're doing) has enough heat energy to stretch the bond (which allows more dissolved salt to fit in between salt molecules), but not energy to break the bonds and become water vapor
example: Like magnets held really far apart but just close enough that they are still in each other's magnetic field. The force holding them together is very weak and there is a lot of space between. (the force attraction in this example is weak because of distance and not because of too much energy, but it is a good visual representation)
(gas) water vapor (aka steam, mist, fog) has too much heat energy. It has so much heat energy that the molecules are so energetic that they vibrate and bounce around until they break free of the bonds that hold them to each other.
Example: Drop a hundred bouncy balls off the roof of a building and watch them bounce all over the place, totally ignoring all the other balls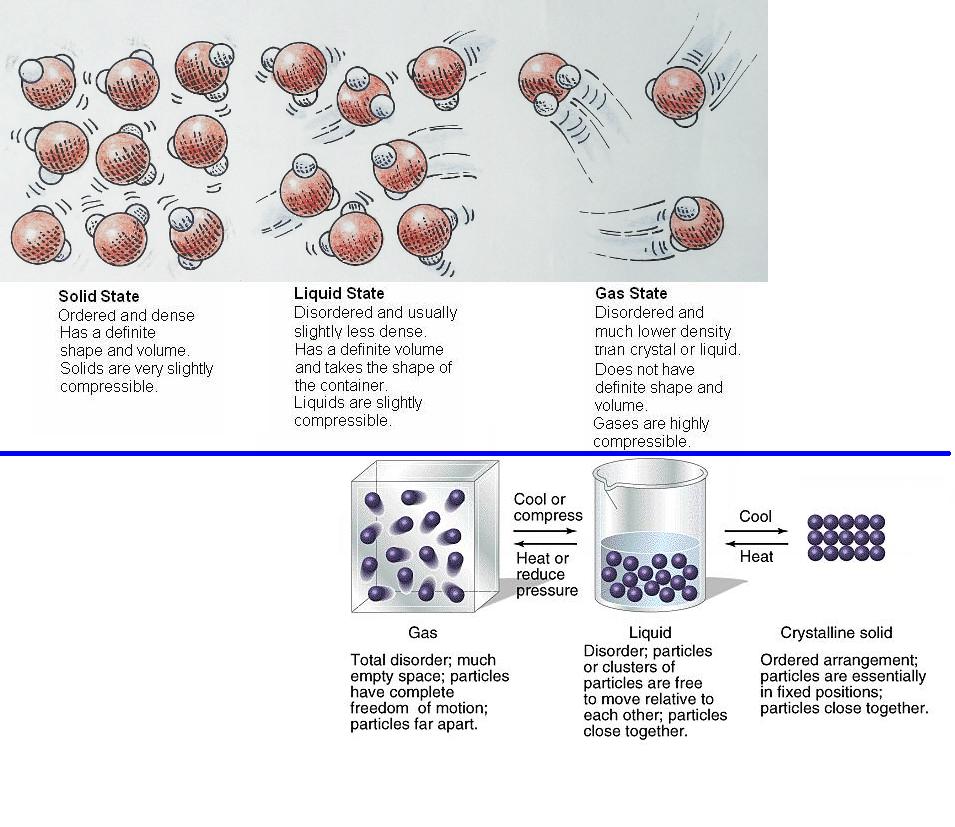
Water that contains undissolved salt = saltwater with a pile of salt in the bottom of the bucket
couldn't you carefully pour most of the water out and when the water level is near the bottom, maybe scoop more water out with a cup. When the bucket has as little water as you can possibly have in it without removing the salt at the bottom, then you put the bucket in the sun and/or in front of a fan to evaporate the remaining water.
Then you scrape the salt off the bottom of the bucket and measure it, either for volume with milliliters in a graduated cylinder or for mass in grams on a scale.
Temperature affects the amount of salt that water can dissolve because:
At high temperatures, the heat energy causes the water molecules to vibrate and bounce around so much that extra space is created between the water molecules.
Frozen solid water (aka ice) has no heat energy. A lack of heat energy means the molecules do not have any energy to move around. They are tightly packed together.
Example: It's like dropping an empty cardboard box (nothing really happens)
room temperature liquid water - has very little heat energy. Its molecules hold themselves near each other and have a little space for dissolved salt to fit in.
Example: Like magnets, you can put several pieces of paper between them and their magnetic field is unaffected. (No difference)
heated liquid water (aka what you're doing) has enough heat energy to stretch the bond (which allows more dissolved salt to fit in between salt molecules), but not energy to break the bonds and become water vapor
example: Like magnets held really far apart but just close enough that they are still in each other's magnetic field. The force holding them together is very weak and there is a lot of space between. (the force attraction in this example is weak because of distance and not because of too much energy, but it is a good visual representation)
(gas) water vapor (aka steam, mist, fog) has too much heat energy. It has so much heat energy that the molecules are so energetic that they vibrate and bounce around until they break free of the bonds that hold them to each other.
Example: Drop a hundred bouncy balls off the roof of a building and watch them bounce all over the place, totally ignoring all the other balls
